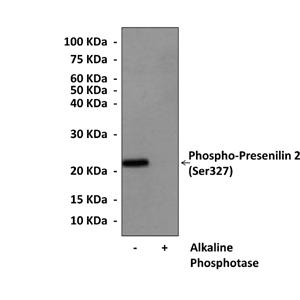
Anti-Phospho-Presenilin 2: Rabbit Presenilin 2, Phospho-Ser327 Antibody |
 |
BACKGROUND Presenilin (PS) is a critical component of the gamma-secretase. Of the two mammalian PS gene homologues, PS1 and PS2, PS1 encodes the major form (PS1) in active gamma-secretase. Nascent PSs undergo endoproteolytic cleavage to generate an amino-terminal fragment (NTF) (29 kDa) and a carboxyl-terminal fragment (CTF) (18 kDa) to form a functional PS heterodimer. Based on observations that PSs possess two highly conserved aspartate residues indispensable for gamma-secretase activity and that specific transition state analogue gamma-secretase inhibitors bind to PS1 NTF/CTF heterodimers, PSs are believed to be the catalytic component of the gamma-secretase complex. PS assembles with three other components, NCT, APH-1, and PEN-2, to form the functional gamma-secretase.1 Strong evidence suggests that PS1/gamma-secretase resides principally in the ER, early Golgi, TGN, endocytic and intermediate compartments, most of which (except the TGN) are not major subcellular sites for beta-amyloid precursor protein (APP). In addition to generating beta-amyloid peptides (Abeta) and cleaving APP to release the APP intracellular domain, PS1/gamma-secretase cleaves other substrates such as Notch, cadherin, ErbB4, and CD44, releasing their respective intracellular domains. Interestingly, PS1/gamma-secretase cleavage of different substrates seems to occur at different subcellular compartments; APP is mainly cleaved at the TGN and early endosome domains, whereas Notch is predominantly cleaved at the cell surface. Thus, perturbing intracellular trafficking of PS1/gamma-secretase may alter interactions between PS1/gamma-secretase and APP, contributing to either abnormal A-beta generation and AD pathogenesis or decreased access of PS1/gamma-secretase to APP such that Abeta production is reduced.2
In addition to participating in gamma-secretase activity, PS1 regulates intracellular trafficking of several membrane proteins, including other gamma-secretase components (nicastrin, APH-1, and PEN-2) and the substrate APP. Intracellular APP trafficking is highly regulated and requires other factors such as mint family members and SorLA. Moreover, it was shown that phospholipase D1 (PLD1) can interact with PS1, and can regulate budding of APP-containing vesicles from the TGN and delivery of APP to the cell surface. Interestingly, It was identified an axonal membrane compartment that contains APP, BACE1, and PS1 and showed that fast anterograde axonal transport of this compartment is mediated by APP and kinesin-I, implying a traffic-regulating role for APP. Increased APP expression is also shown to decrease retrograde axonal transport of nerve growth factor. It was demonstrated that intracellular trafficking of PS1, as well as that of other gamma-secretase components, but not BACE1, is regulated by APP. APP deficiency promotes cell surface delivery of PS1/gamma-secretase complex and facilitates PS1/gamma-secretase-mediated Notch cleavage.3 In addition, PLD1 also regulates intracellular trafficking of PS1 through a different mechanism and more potently than APP.4
Recent studies in fibroblast cells implicate PS1 in the regulation of the PI3K/Akt signaling. It was shown that the onset of neuronal maturation coincides with a decline in the ability of PS1 null neurons to support PI3K/Akt signaling resulting in impaired phosphorylation/inactivation of GSK-3beta, increased activation of apoptotic caspase-3 and progressive neurodegeneration. Importantly, PS1 FAD mutations inhibit the functions of PS1 in PI3K/Akt signaling and promote apoptosis, which contributes to neurodegeneration.5 In addition, PS2 is cleaved by caspases during apoptosis between Asp-329 and Ser-330. Caspase-mediated cleavage of this molecule was abrogated by Ser327 and Ser330 phosphorylation inside the consensus cleavage site DSYDS.6
REFERENCES
In addition to participating in gamma-secretase activity, PS1 regulates intracellular trafficking of several membrane proteins, including other gamma-secretase components (nicastrin, APH-1, and PEN-2) and the substrate APP. Intracellular APP trafficking is highly regulated and requires other factors such as mint family members and SorLA. Moreover, it was shown that phospholipase D1 (PLD1) can interact with PS1, and can regulate budding of APP-containing vesicles from the TGN and delivery of APP to the cell surface. Interestingly, It was identified an axonal membrane compartment that contains APP, BACE1, and PS1 and showed that fast anterograde axonal transport of this compartment is mediated by APP and kinesin-I, implying a traffic-regulating role for APP. Increased APP expression is also shown to decrease retrograde axonal transport of nerve growth factor. It was demonstrated that intracellular trafficking of PS1, as well as that of other gamma-secretase components, but not BACE1, is regulated by APP. APP deficiency promotes cell surface delivery of PS1/gamma-secretase complex and facilitates PS1/gamma-secretase-mediated Notch cleavage.3 In addition, PLD1 also regulates intracellular trafficking of PS1 through a different mechanism and more potently than APP.4
Recent studies in fibroblast cells implicate PS1 in the regulation of the PI3K/Akt signaling. It was shown that the onset of neuronal maturation coincides with a decline in the ability of PS1 null neurons to support PI3K/Akt signaling resulting in impaired phosphorylation/inactivation of GSK-3beta, increased activation of apoptotic caspase-3 and progressive neurodegeneration. Importantly, PS1 FAD mutations inhibit the functions of PS1 in PI3K/Akt signaling and promote apoptosis, which contributes to neurodegeneration.5 In addition, PS2 is cleaved by caspases during apoptosis between Asp-329 and Ser-330. Caspase-mediated cleavage of this molecule was abrogated by Ser327 and Ser330 phosphorylation inside the consensus cleavage site DSYDS.6
REFERENCES
1. Mattson, M.P. & Guo, Q. : Neuroscient. 5:112-24, 1999
2. Suh, Y-H. & Checler, F.:Pharmacol. Rev. 54:469-525, 2002
3. Checler, F.: J. Neurochem. 76:1621-27, 2001
4. Cai, D. et al: Proc. Natl. Acad. Sci. USA 103:1941-6, 2006
5. Pap, M. & Cooper, G.M.: Mol. Cell. Biol. 22:578-86, 2002
6. Walter, J. et al: Proc. Natl. Acad. Sci. USA 96:1391-6, 1999
2. Suh, Y-H. & Checler, F.:Pharmacol. Rev. 54:469-525, 2002
3. Checler, F.: J. Neurochem. 76:1621-27, 2001
4. Cai, D. et al: Proc. Natl. Acad. Sci. USA 103:1941-6, 2006
5. Pap, M. & Cooper, G.M.: Mol. Cell. Biol. 22:578-86, 2002
6. Walter, J. et al: Proc. Natl. Acad. Sci. USA 96:1391-6, 1999
Products are for research use only. They are not intended for human, animal, or diagnostic applications.
Параметры
|
Cat.No.:
|
CG1480
|
|
Antigen:
|
Short peptide from human Presenilin 2 sequence surrounding and containing phospho-Ser327.
|
|
Isotype:
|
Rabbit IgG
|
|
Species & predicted
species cross-
reactivity ( ):
|
Human, Mouse, Rat
|
|
Applications &
Suggested starting
dilutions:*
|
WB 1:1000 - 1:5000
IP 1:50
IHC n/d
ICC n/d
FACS n/d
|
|
Predicted Molecular
Weight of protein:
|
18 - 23 kDa
|
|
Specificity/Sensitivity:
|
Detects endogenous phosphorylated Presenilin 2 (Ser327) proteins without cross-reactivity with other family members.
|
|
Storage:
|
Store at -20°C, 4°C for frequent use. Avoid repeated freeze-thaw cycles.
|
*Optimal working dilutions must be determined by end user.
Информация представлена исключительно в ознакомительных целях и ни при каких условиях не является публичной офертой